Rockland社は、米国のウィスター研究所と提携し、メラノーマ細胞株の販売を開始致しました。
患者の転移組織に由来する低継代の細胞株で、BRAF、N-RAS、KIT、PTEN、CDK4等の遺伝子変異を確認しております。100種類以上の細胞株をラインアップしており、遺伝学研究、創薬研究、薬剤探索、ゼノグラフトモデルの作製に有用です。
また、各細胞株由来のライセート、抽出ゲノムDNA/トータルRNAも提供しております。
Rockland社は、米国のウィスター研究所と提携し、メラノーマ細胞株の販売を開始致しました。
患者の転移組織に由来する低継代の細胞株で、BRAF、N-RAS、KIT、PTEN、CDK4等の遺伝子変異を確認しております。100種類以上の細胞株をラインアップしており、遺伝学研究、創薬研究、薬剤探索、ゼノグラフトモデルの作製に有用です。
また、各細胞株由来のライセート、抽出ゲノムDNA/トータルRNAも提供しております。

腫瘍中の細胞や分子は「不均一性(heterogeneity)」を有することがよく知られており、異なる腫瘍細胞はそれぞれ異なる表現型と原発がん由来の分子的特徴(染色体コピー数変異、一塩基多型、遺伝子発現特性)を示します。
この不均一性は、治療において患者特異的な応答を生じさせるため、臨床や癌治療薬の開発で課題となっています。また、標的治療薬等に代表されるprecision medicine(個別化医療)の登場で、抗癌治療に応答する腫瘍亜集団の特定がますます重要になっています。そのため患者の腫瘍特性をより正確に反映する腫瘍モデルの構築が必要とされており、それらは新規抗癌剤の試験への応用が期待されます。
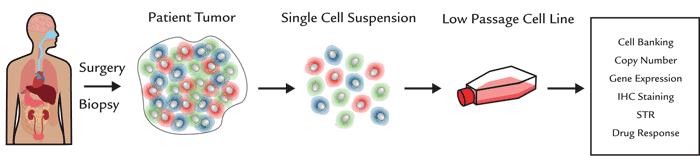
図1. メラノーマ細胞株の開発。
細胞は低回数の継代後、保存および検証(コピー数、遺伝子発現解析、既知腫瘍マーカーの免疫組織染色、STR解析、薬剤応答を含む)されます。
Rockland Introduces Melanoma Cell Lines in Collaboration with The Wistar Institute
ウィスター研究所のDr. Meenhard Herlynによって提供されるメラノーマ細胞株は、mRNAのqPCR、DNA STR(short tandem repeat)解析を行って特徴づけされています。
メラノーマ細胞株は、少なくとも5つの遺伝子(BRAF、N-RAS、KIT、PTEN、CDK4)の遺伝子型(野生型/変異型)を確認しています。
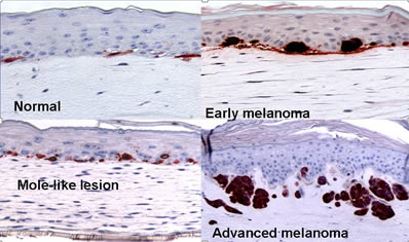
図2. 皮膚合成におけるメラノーマ形成
(Image courtesy of Dr. Meenard Herlyn, Wistar Institute, Philadelphia, PA.)

図3. メラノーマの微小環境
(Image courtesy of Dr. Meenard Herlyn, Wistar Institute, Philadelphia, PA.)
| Cell Lines | Disease Stage | BRAF | PTEN | N-RAS | c-KIT | CDK4 | OTHER | Age /Gender | Pathology | Site |
|---|
| WM1552C-01-0001 | Primary/RGP | V600E | Mutated/ Hemizygous Deletion | WT | WT | WT | TP53 | 72/Male | Superficial Spreading Melanoma (SSM), Stage 3 | Buttock |
| 451Lu-01-0001 | Xenograft Metastasis | V600E | WT | WT | WT | WT | TP53, CDKN2A, ROS1 | 22/Male | Superficial Spreading Melanoma (SSM), stage IV tumor | Lung |
| 451lLu BR-01-0001 | BRAF Resistant | V600E | ND | WT | WT | WT | 22/Male | Superficial Spreading Melanoma (SSM), stage IV tumor | Lung | |
| WM164-01-0001 | Metastasis | V600E | WT | WT | WT | WT | TP53, CDKN2A | 22/Male | Superficial Spreading Melanoma (SSM), Level IV, Thickness 1.72 mm | Right Upper Arm |
| WM1716-01-0001 | Metastasis | V600E | ND | WT | WT | WT | ||||
| WM1727A-01-0001 | Metastasis | V600E | Hemizygous Deletion | WT | WT | WT | ATM, PPP6C, CDKN2A, MAP2K2, Y134H | |||
| WM1799-01-0001 | Metastasis | V600E | Hemizygous Deletion | WT | WT | WT | ARID2 | |||
| WM1819-01-0001 | Unknown | V600E | ND | WT | WT | WT | TRRAP, TP53 | |||
| WM1852-01-0001 | Metastasis | V600E | WT | WT | WT | |||||
| WM1862-01-0001 | Primary/RGP | V600E | ND | WT | WT | WT | ||||
| WM1960-01-0001 | Metastasis | WT | WT | Q61K | WT | WT | 55/Male | Level III malignant melanoma, VGP and RGP with a tumor thickness of 3.0 mm | Left Axillary Nodes | |
| WM2013-01-0001 | Metastasis | WT | WT | Q61K | WT | WT | 55/Male | Level III malignant melanoma, VGP and RGP with a tumor thickness of 3.0 mm | Left Axillary Nodes | |
| WM3060-01-0001 | Metastasis | WT | WT | Q61K | WT | WT | CDKN2A, MLH1, RAC1 | 55/Male | Level III malignant melanoma, VGP and RGP with a tumor thickness of 3.0 mm | Left Axillary Nodes |
| WM1963-01-0001 | Unknown | N581S | WT | A146T | WT | WT | CTNNB1, NF1, TP53, KDR, MAP2K1 HOM | 56/Male | ||
| WM1985-01-0001 | Unknown | V600E | Homozygous Deletion | WT | WT | WT | TP53 | |||
| WM2032-01-0001 | Metastasis | WT | WT | Q61R | WT | WT | Lymph Node | |||
| WM3000-01-0001 | Metastasis | WT | WT | Q61R | WT | WT | ||||
| WM2044-01-0001 | Metastasis | V600E | Hemizygous Deletion | WT | WT | WT | TP53 | |||
| WM209-01-0001 | Metastasis | WT | Homozygous Deletion | G12A | WT | WT | ||||
| WM2090-01-0001 | Metastasis | V600E | WT | WT | WT | WT | CDKN2A, DDDX3X | |||
| WM262-01-0001 | Metastasis | V600E | ND | WT | WT | WT | ||||
| WM3061-01-0001 | Metastasis | ND | WT | G13R | ND | ND | ||||
| WM3246-01-0001 | Metastasis | WT | Mutated | WT | WT | WT | Female | Adrenal | ||
| WM3268-01-0001 | Primary/VGP | WT | ND | Q61K | WT | WT | ARID1A, ARID1B | |||
| WM3282-01-0001 | Primary/VGP | V600K | WT | WT | WT | WT | DCC, ERBB4, NTRK1, TP53 | |||
| WM3301-01-0001 | Metastasis | V600E | Homozygous Deletion | WT | WT | WT | ||||
| WM3311-01-0001 | Metastasis | WT | WT | WT | WT | WT | 34/Female | Lymph Node | ||
| WM3406-01-0001 | Metastasis | WT | Mutated | Q61K | WT | WT | ||||
| WM3438-01-0001 | Metastasis | WT | ND | WT | WT | WT | ||||
| WM3456-01-0001 | Metastasis | WT | WT | Q61K | WT | WT | ||||
| WM3482-01-0001 | Metastasis | V600E | WT | WT | WT | WT | ||||
| WM3506-01-0001 | Metastasis | WT | WT | Q61R | WT | WT | Lymph Node | |||
| WM3533-01-0001 | Metastasis | V600E | ND | WT | WT | WT | ARID2 | Lymph Node | ||
| WM3540-01-0001 | Metastasis | V600E | ND | WT | WT | D97H | Lymph Node | |||
| WM3619-01-0001 | Metastasis | WT | WT | Q61R | WT | WT | ROS1 | |||
| WM3622-01-0001 | Metastasis | WT | Mutated | WT | WT | WT | ||||
| WM3623-01-0001 | Metastasis | WT | WT | Q61K | WT | WT | ARID2 | 48/Male | Right Lymph Node Resection, Level 5: Metastatic Malignant Melanoma in Two of Eight Lymph Nodes | Right Neck Lymph Nodes |
| WM3629-01-0001 | Metastasis | D549G | WT | G12D | WT | WT | Lymph Node | |||
| WM3630-01-0001 | Metastasis | V600E | ND | WT | WT | C70T | TP53 | |||
| WM3681-01-0001 | ||||||||||
| WM3682-01-0001 | Metastasis | WT | WT | Q61L | WT | WT | 58/Male | Necrotic Fungating Melanoma, Right Flank: Metastatic Melanoma Forming Multiple Subcutaneous Nodules, with Overlying Skin Ulceration | Right Upper Flank of Back | |
| WM3702-01-0001 | Metastasis | WT | ND | Q61L | WT | WT | FBXW7 | 58/Male | Necrotic Fungating Melanoma, Right Flank: Metastatic Melanoma Forming Multiple Subcutaneous Nodules, with Overlying Skin Ulceration | Right Upper Flank of Back |
| WM3758-01-0001 | Metastasis | WT | ND | Q61L | WT | WT | 58/Male | Necrotic Fungating Melanoma, Right Flank: Metastatic Melanoma Forming Multiple Subcutaneous Nodules, with Overlying Skin Ulceration | Right Upper Flank of Back | |
| WM3704-01-0001 | Metastasis | V600E | Mutated | WT | WT | R24H | TP53 | |||
| WM373-01-0001 | Metastasis | V600E | WT | WT | WT | WT | CDKN2A | 49/Male | Superficial Spreading Melanoma (SSM), Level IV, 3.78 mm Thickness, 3 Cell Types in VGP | Left Upper Back |
| WM3743-01-0001 | Primary Post Chemotherapy | WT | ND | WT | WT | WT | ARD12, CDKN2A, DCC, TP53 | |||
| WM3749-01-0001 | Metastasis | V600E | Homozygous Deletion | WT | WT | WT | 54/Female | |||
| WM3755-01-0001 | Metastasis | V600E | ND | WT | WT | WT | ||||
| WM3618F-01-0001 | Primary, Metastasis of UVEAL | WT | ND | WT | WT | WT | GNAQ, BAP1, CTNNB1 | Lymph Node | ||
| WM3772F-01-0001 | Primary Metastasis of UVEAL | WT | ND | WT | WT | WT | GNAQ, BAP1 | |||
| WM3792-01-0001 | Metastasis | V600K 601 deletion | Homozygous Deletion | WT | WT | WT | ||||
| WM47-01-0001 | Metastasis | V600E | ND | WT | WT | WT | ||||
| WM51-01-0001 | Metastasis | V600E | WT | WT | WT | WT | Male | |||
| WM75-01-0001 | Metastasis | V600E | WT | WT | WT | WT | ||||
| WM8-01-0001 | Metastasis | WT | WT | WT | N818K | WT | ||||
| WM853-2-01-0001 | Primary/VGP | V600E | WT | WT | WT | WT | CDKN2A | 71/Male | Back | |
| WM858-01-0001 | Metastasis | V600E | WT | WT | WT | WT | 48/Female | Malignant Melanoma, Superficial Spreading Melanoma (SSM), Polypoid VGP, Level III, 3.24 mm | Right Axillary Nodes | |
| WM873-1-01-0001 | Metastasis | V600E | Hemizygous Deletion | WT | WT | WT | ||||
| WM873-2-01-0001 | Metastasis | ND | Hemizygous Deletion | ND | ND | ND | ||||
| WM989-01-0001 | Unknown | V600E | ND | WT | WT | WT | CDKN2A, PIK3CA, TP53, ROS1 | |||
| WM3912-01-0001 | Metastasis | N581Y | WT | WT | WT | L213F | Neck | |||
| WM3918-01-0001 | Metastasis | WT | ND | WT | WT | WT | PPP6C | |||
| WM4002-01-0001 | Metastasis | V600E | Hemizygous Deletion | WT | WT | WT | Lymph Node | |||
| WM1026-01-0001 | Metastasis | V600E | WT | WT | WT | WT | Lymph Node | |||
| WM1232-01-0001 | Metastasis | V600E | Hemizygous Deletion | WT | WT | WT | TP53, CDKN2A | |||
| WM902B-01-0001 | VGF | V600E | Hemizygous Deletion | WT | WT | R24L | AKT1 | |||
| WM115-01-0001 | VGF | V600D | Hemizygous Deletion | WT | WT | WT | 55/Female | Superficial Spreading Melanoma (SSM), VGP type with Clark Level III tumor, 2.24 mm Thickness | Right Anterior Leg | |
| WM165-1-01-0001 | Metastasis | V600D | Hemizygous Deletion | WT | WT | WT | 55/Female | Superficial Spreading Melanoma (SSM), VGP type with Clark Level III tumor | Lymph Node Metastasis | |
| WM239A-01-0001 | Metastasis | V600D | Hemizygous Deletion | WT | WT | WT | 55/Female | Superficial Spreading Melanoma (SSM), VGP type with Clark Level III tumor | Lymph Node Metastasis | |
| WM266-4-01-0001 | Metastasis | V600D | Hemizygous Deletion | WT | WT | WT | 55/Female | Superficial Spreading Melanoma (SSM), VGP type with Clark Level III tumor | Lymph Node Metastasis | |
| 1205 Lu-01-0001 | Metastasis | V600E | Mutated/ Hemizygous Deletion | WT | WT | K22Q | 37/Male | Lung | ||
| WM1158-01-0001 | Metastasis | V600E | Hemizygous Deletion | WT | WT | WT | CDKN2A | |||
| WM1341D-01-0001 | VGF | V600R | WT | WT | WT | WT | ||||
| WM1361A-01-0001 | VGF | WT | Hemizygous Deletion | Q61R Homozygous | WT | WT | PTCH1 | |||
| WM1366-01-0001 | VGF | WT | WT | Q61L | WT | WT | DDX3X | 79/Male | Superficial Spreading Melanoma (SSM), Stage IV tumor | Right Forearm |
| WM1789-01-0001 | Unknown | K601E Homozygous, S363F | Hemizygous Deletion | WT | WT | WT | MAP2K2, E207R | |||
| WM1791C-01-0001 | Metastasis | WT | ND | WT (KRAS T50I, E62K) | WT | K22Q | RAC1, KRAS, T501, E62K | |||
| WM278-01-0001 | VGF | V600E | Hemizygous Deletion | WT | WT | WT | ROS1 | |||
| WM3211-01-0001 | VGF | WT | WT | WT | L576P | WT | CDKN2A, ARID1A, TP53 | 34/Female | Lymph Node | |
| WM3248-01-0001 | Unknown | V600E | Mutated/ Hemizygous Deletion | WT | WT | WT | ||||
| WM3670-01-0001 | Metastasis | G469E | WT | G12D | WT | WT | ||||
| WM3734-01-0001 | Metastasis | V600E | ND | WT | WT | WT | 24/Female | Brain | ||
| WM39-01-0001 | VGF | V600E | WT | WT | WT | R24C | ||||
| WM46-01-0001 | Metastasis | V600E | Hemizygous Deletion | WT | WT | R24C | AKT3, STK11 | 43/Female | Superficial Spreading Melanoma (SSM), Level IV with a tumor thickness of 1.44 mm | Left Chest and Abdomen |
| WM852-01-0001 | Metastasis | V600E | Hemizygous Deletion | Q61R | WT | WT | TP53 | 63/Male | Abdomen | |
| WM88-01-0001 | Metastasis | V600E | WT | WT | WT | WT | Male | Superficial Spreading Melanoma (SSM), Level III with a tumor thickness of 0.92 mm | ||
| WM9-01-0001 | Metastasis | V600E | Hemizygous Deletion | WT | WT | WT | Male | Left Axillary Node | ||
| WM983A-01-0001 | VGF | V600E | WT | WT | WT | WT | TP53 | 54/Male | Malignant Melanoma, Polypoid type, Level IV, 2.5 cm | Inguinal Node Dissection |
| WM983B-01-0001 | Metastasis | V600E | WT | WT | WT | WT | TP53, CDKN2A | 54/Male | Malignant Melanoma, Polypoid type, Level IV, 2.5 cm | Inguinal Node Dissection |
| WM983BBR-01-0001 | BRAF Resistant | V600E | ND | WT | WT | WT | 54/Male | Malignant Melanoma, Polypoid type, Level IV, 2.5 cm | Inguinal Node Dissection | |
| WM983C-01-0001 | Metastasis | V600E | WT | WT | WT | WT | 54/Male | Malignant Melanoma, Polypoid type, Level IV, 2.5 cm | Inguinal Node Dissection | |
| WM3854-01-0001 | Metastasis | WT | ND | Q61L | WT | WT | 64/Male | Highly pigmentic metastasis with superficial and deep inguinal nodes forming a 3.0 cm mass | Right Upper Flank of Back | |
| WM3928-01-0001 | Metastasis | WT | Y177X | WT | WT | WT | Left Inguinal Lymph Node |
Human Melanoma Cell Lines商品のご購入には、End-User License Agreement ![]() が必要となります。ご注文の際に、必要事項をご記入の上、注文書と一緒に弊社商品取扱い代理店へお送り下さい。
が必要となります。ご注文の際に、必要事項をご記入の上、注文書と一緒に弊社商品取扱い代理店へお送り下さい。
尚、本商品は研究目的でご使用されるアカデミックユーザー様のみに販売しております。営利団体および企業ユーザー様は弊社営業部までお問い合わせいただきますようお願い申し上げます。
商品は「研究用試薬」です。人や動物の医療用・臨床診断用・食品用としては使用しないように、十分ご注意ください。
※ 表示価格について
© COSMO BIO